
Baby's Cabaret
Baby's Cabaret is one of Toronto's best places for a girl's night out. What makes it so special?
Have you ever seen the 2010 film Burlesque starring Cher and Christina Aguilera? Well imagine that but more leopard print, more feathers and more poles.
Starting off with its central location near Queen and Bathurst, Baby's Cabaret makes for a fun stop along the Queen West strip. It's also just a short jaunt up Portland from the bars on King West.

A dancer in front of a mural designed by Candice Kaye.
Inside, walls have been decorated by local Toronto artist Candice Kaye who has done work for other businesses in Toronto before. Her work isn't hard to miss due to its unique, tasteful, and space elevating nature. You can find it at places like Planta on Queen, Maman, Byblos Uptown, and now Baby's Cabaret.
The fun and colourful decor isn't just for looks, it's also an extention of Baby's Cabaret's goals.
"These days with housing prices skyrocketing and the economy of Toronto, people, if they are going to spend their hard earned money, aren't just looking to go to a bar and entertain themselves anymore," says Baby's Cabaret General Manager Emma Stairs.
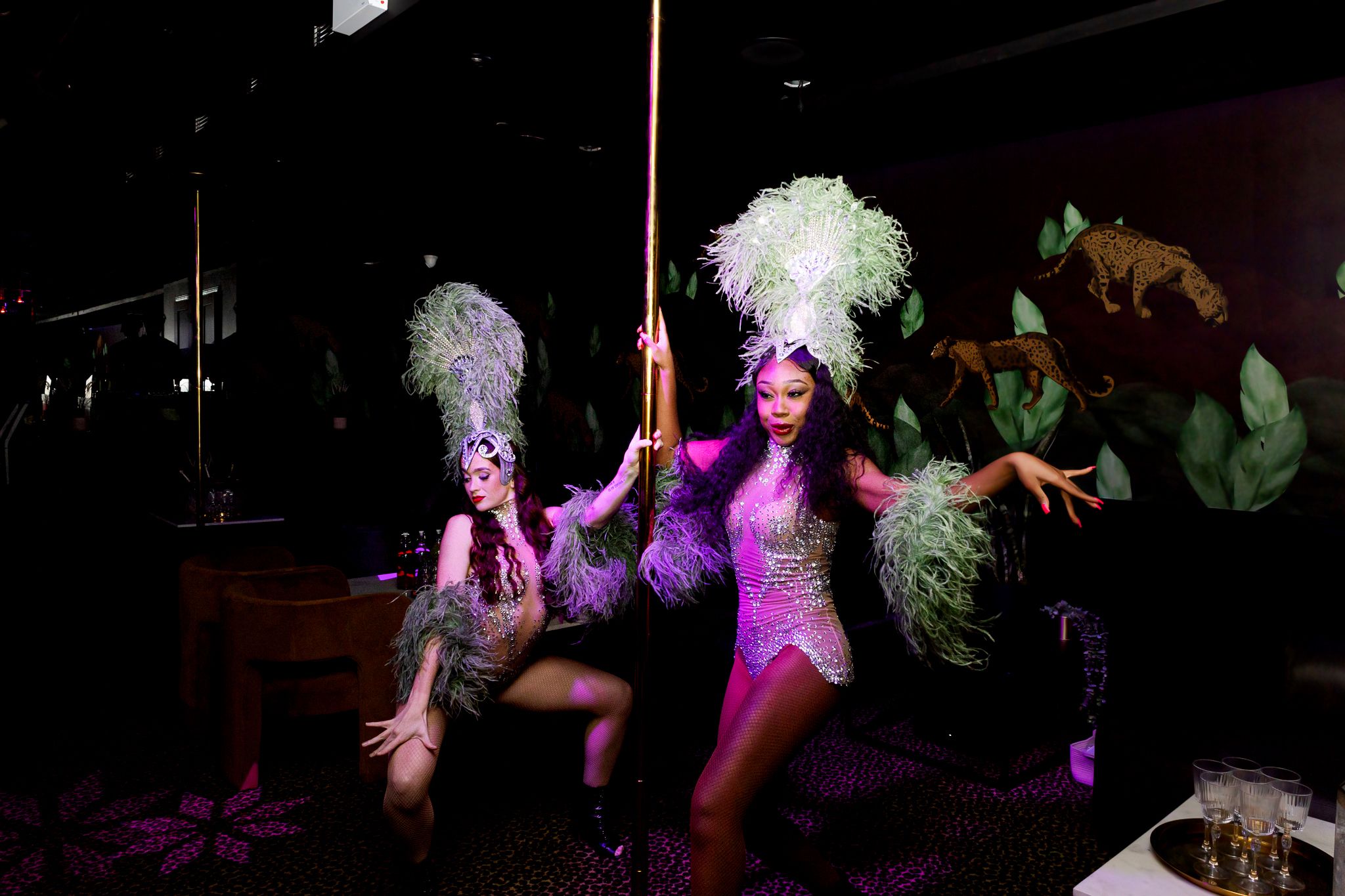 "Here (Baby's Cabaret), the dancing is the focus. We are bringing the entertainment to you."
"Here (Baby's Cabaret), the dancing is the focus. We are bringing the entertainment to you."
In partnership with Medusa Entertainment, a company that curates entertainment for venues and events all over the city, Baby's Cabaret has created a night out experience that's different from most other spots.
Eva Mok, the Creative Director of Medusa Entertainment, was a dancer herself and has been a pioneer in the her respective industry.

Rachael Pazan of Medusa Entertainment, along with two of the dancers.
"We invite people of all different backgrounds, ethnicity, shape and size to be a part of our company and work with us," says Mok.
"Of course the stigma is something that we consider. With every residency we take the owners vision while also actively trying to educate them. Diversity, inclusivity, and appropriation. We make sure our entertainers are treated the way they should be."
 Pole dancing, Stripping, and GoGo dancing have been some of the dance styles that have recieved negative stigma for what many have said are forms of over sexualizing women.
Pole dancing, Stripping, and GoGo dancing have been some of the dance styles that have recieved negative stigma for what many have said are forms of over sexualizing women.
In the last few years we have seen a rise in women wanting to partake in pole dancing in a way of reclaiming their bodies and wanting to feel sexy.
 "Women are realizing that they can take back their power in their sexuality through having control in what they do with it," says dancer Rachael Pazan.
"Women are realizing that they can take back their power in their sexuality through having control in what they do with it," says dancer Rachael Pazan.
"Gogo dancing was a choice for me, and I feel so powerful doing it. I know how it makes me feels, I'm in control, I decide what comes off, and I decide how far the interactions go."
 At Baby's Cabaret, you can expect to find good cocktails alongside dancing performances all night long, or every 15-30 minutes.
At Baby's Cabaret, you can expect to find good cocktails alongside dancing performances all night long, or every 15-30 minutes.
Dancers go from wall to wall, and believe it or not, floor to ceiling. They climb poles using an incredible amount of core strength, then spinning down, hopping on tables, dancing on the bar and maybe even the windows.
 You can expect dancers to come right up and interact with you throughout their peformance.
You can expect dancers to come right up and interact with you throughout their peformance.
"I started dancing as a kid because of the movie Burlesque so dancing at Baby's (Cabaret) is a full circle moment for me," says Pazan, "You get to live your creative freedom. It's an added perk to getting to be sexy, strong, and finding your feminine while exuding your sexuality in a healthy way."
 Many of the routines are choreographed by the dancers themselves and include an amazing amount of improvisation as they never know when a server might walk around with a tray full of drinks, causing them to have to switch up their routine so no mishaps occur.
Many of the routines are choreographed by the dancers themselves and include an amazing amount of improvisation as they never know when a server might walk around with a tray full of drinks, causing them to have to switch up their routine so no mishaps occur.
But don't think that the dancing is only reserved for the professionals!
 Branded Baby's Cabaret slippers are offered to everyone so you can take off your shoes and slip into something comfortable and maybe even dance on top of the couches.
Branded Baby's Cabaret slippers are offered to everyone so you can take off your shoes and slip into something comfortable and maybe even dance on top of the couches.
The poles aren't off limits either, when free you an hop on the pole and try a trick out for yourself. In between routines the dancers are out mingling and can offer a free lesson.
I took my chance with a few spins and got a cheer from the whole bar!
 From the bar menu, cocktails range from $22-$24, a little pricey by Toronto standards. They also offer bottle service which you can book online.
From the bar menu, cocktails range from $22-$24, a little pricey by Toronto standards. They also offer bottle service which you can book online.
"We've curated a list of cocktails that have some sort of relation to the bar, their fruity, fun, and delicious," says Stairs. "The names are fun too!"
 With names like Razzle Dazzle, Lips Like Sugar, and Slippery When Wet, the drinks are definitely on theme.
With names like Razzle Dazzle, Lips Like Sugar, and Slippery When Wet, the drinks are definitely on theme.
Get Nuts, a cocktail made with Johnnie Walker Black Label Scotch, drambuie, feangelico, sortilege, lemon juice, and egg white offers a smoky taste that glides easily every sip.
 Their Baby Blue cocktail made with Plantation 3 Star Rum, Hynotiq, and coconut water is sweet and complements the safari decor.
Their Baby Blue cocktail made with Plantation 3 Star Rum, Hynotiq, and coconut water is sweet and complements the safari decor.  If you aren't an alcohol drinker, that's no problem!
If you aren't an alcohol drinker, that's no problem!
The Tall Dark and Handsome (Made with Captain Morgan spiced gold 0.0) and Baby's Gotta Work (Made with Tanqueray Flor de Sevilla 0.0) are from Baby Cabaret's zero alcohol menu.
 There's also a menu of bar snacks. A platter of pickles, olives and other savoury items is an easy one to share among friends.
There's also a menu of bar snacks. A platter of pickles, olives and other savoury items is an easy one to share among friends. Baby's Cabaret is located at 563 Queen St West and is open on Friday, Saturday and Sunday only. Must be 25+ to enter with a style code in effect
Baby's Cabaret is located at 563 Queen St West and is open on Friday, Saturday and Sunday only. Must be 25+ to enter with a style code in effect
Fareen Karim
Latest Videos
Baby's Cabaret
Baby's Cabaret
1 Upcoming Event
-
January 18
FIESTA - Toronto's Hottest Latin Party on Saturday January 18th inside Baby's (563 Queen Street West, Toronto). Enjoy Reggaeton, Salsa, Bachata, and Brazilian Funk all night long.
Tickets start at only $5. Party starts at 10pm
This is a 19+ event.
For tickets and tables please call 416-557-3032







